Std 10 Science Part 1 Chapter 8 Metallurgy Notes Maharashtra Board
Class 10 Science Part 1 Chapter 8 Metallurgy Notes Maharashtra Board

Physical properties of metals:
- Metals mainly exist in solid state.
- Mercury and gallium are exceptions — they are liquid at room temperature.
- Metals have luster (shine), but this shine decreases over time due to exposure to
oxygen, moisture, and reactive gases. - Metals are ductile — they can be drawn into thin wires.
- Metals are malleable — they can be hammered into thin sheets.
- All metals are good conductors of heat and electricity.
- Generally, metals are hard.
- Alkali metals (like lithium, sodium, and potassium) are soft and can be cut with a
knife. - Metals usually have high melting and boiling points. For example, tungsten has the
highest melting point (3422°C). - Some metals like sodium, potassium, mercury, and gallium have low melting and
boiling points. - When certain metals are struck, they produce a ringing sound — this property is
called sonority. Such metals are called sonorous metals.
Physical properties of non-metals:
- Some nonmetals are in solid state, and some are in gaseous state.
- Bromine is the only nonmetal that exists in liquid state.
- Nonmetals generally do not have luster (shine), but iodine crystals are an exception
— they are shiny. - Nonmetals are generally not hard.
- Diamond (an allotrope of carbon) is an exception — it is the hardest natural
substance. - Nonmetals usually have low melting and boiling points.
- Nonmetals are poor conductors of heat and electricity.
- Graphite (another allotrope of carbon) is an exception — it conducts electricity well.
Chemical properties of metals:
- Metals are reactive.
- They lose electrons easily and become positively charged ions.
- That is why metals are called electropositive elements.
Do you know?
- Substances which are good conductors of heat are usually good conductors of
electricity as well. - Similarly bad conductors of heat are also bad conductors of electricity.
- The exception is diamond which is bad conductor of electricity but good conductor
of heat.

Reactions of Metals:
a. Reaction of metals with oxygen
- Metals combine with oxygen when heated in air, forming metal oxides.
- Sodium and potassium are very reactive metals.
- Sodium reacts with oxygen even at room temperature to form sodium oxide:
4Na(s) + O₂(g) → 2Na₂O(s) - Sodium easily catches fire when exposed to air, so it is stored in kerosene for safety.
- Some metal oxides dissolve in water and form alkali (basic solutions).
Example:
Na₂O(s) + H₂O(l) → 2NaOH(aq) - Burning a magnesium ribbon in air produces magnesium oxide:
2Mg(s) + O₂(g) → 2MgO(s) - Magnesium oxide reacts with water to form magnesium hydroxide (an alkali):
MgO + H₂O → Mg(OH)₂
b. Reaction of metals with water
- Sodium and potassium react very rapidly and vigorously with water, releasing
hydrogen gas and heat:
o 2Na(s) + 2H₂O(l) → 2NaOH(aq) + H₂(g) + heat
o 2K(s) + 2H₂O(l) → 2KOH(aq) + H₂(g) + heat - Calcium reacts slowly and less vigorously with water. The hydrogen gas forms
bubbles on the metal’s surface, making the metal float:
o 2Ca(s) + 2H₂O(l) → 2Ca(OH)₂(aq) + H₂(g) - Aluminium, iron, and zinc do not react with cold or hot water but react with steam
to form metal oxides and release hydrogen gas:
o 2Al(s) + 3H₂O(g) → Al₂O₃(s) + 3H₂(g)
o 3Fe(s) + 4H₂O(g) → Fe₃O₄(s) + 4H₂(g)
o Zn(s) + H₂O(g) → ZnO(s) + H₂(g)
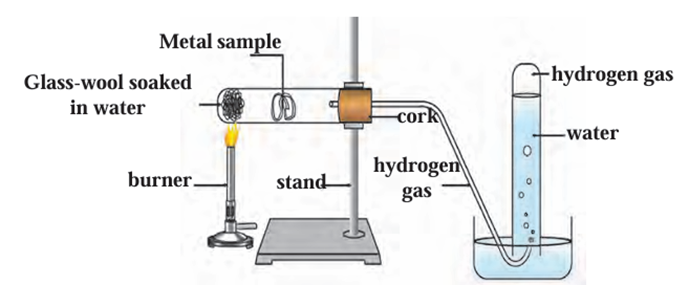
Reaction of a metal with water
c. Reaction of metals with oxygen
- Not all metals are equally reactive with acids.
- When aluminium, magnesium, iron, or zinc react with dilute sulphuric acid or
hydrochloric acid, they form metal salts (sulphates or chlorides) and release
hydrogen gas. - The reactivity order of these metals is:
Magnesium (Mg) > Aluminium (Al) > Zinc (Zn) > Iron (Fe) - Example reactions:
o Magnesium:
Mg(s) + 2HCl(aq) → MgCl₂(aq) + H₂(g)
o Aluminium:
2Al(s) + 6HCl(aq) → 2AlCl₃(aq) + 3H₂(g)
o Zinc:
Zn(s) + 2HCl(aq) → ZnCl₂(aq) + H₂(g)
o Iron:
Fe(s) + 2HCl(aq) → FeCl₂(aq) + H₂(g)
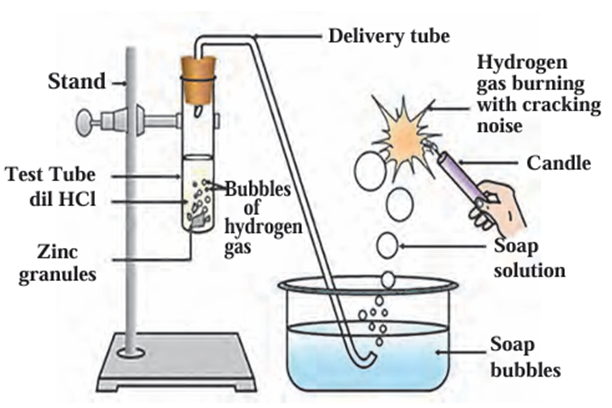
Reaction of metals with dilute acid
d. Reaction of metals with nitric acid
- When metals react with nitric acid, they form nitrate salts.
- Depending on the concentration of nitric acid, different nitrogen oxides are
produced (like N₂O, NO, NO₂). - Example reactions with copper (Cu):
o With concentrated nitric acid:
Cu(s) + 4HNO₃(aq) → Cu(NO₃)₂(aq) + 2NO₂(g) + 2H₂O(l)
o With dilute nitric acid:
3Cu(s) + 8HNO₃(aq) → 3Cu(NO₃)₂(aq) + 2NO(g) + 4H₂O(l)
Aqua Regia:
- Aqua regia is a highly corrosive and fuming liquid.
- It is one of the few reagents which can dissolve the noble metals like gold and
platinum. - Aqua regia is freshly prepared by mixing concentrated hydrochloric acid and
concentrated nitric acid in the ratio 3:1.
e. Reaction of metals with salts of other metals
a. In which test tube has a reaction taken place?
The reaction takes place in the test tube where the iron nail is dipped in copper
sulphate solution.
b. How did you recognize that a reaction has taken place?
- You observe that a reddish-brown layer (copper) forms on the surface of the iron
nail. - The blue color of the copper sulphate solution slowly fades because copper ions are
being displaced.
c. What is the type of the reaction?
This is a displacement reaction (also called a single displacement reaction), where a
more reactive metal (iron) displaces a less reactive metal (copper) from its
compound.
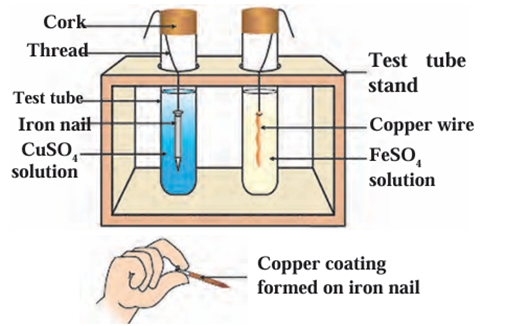
Reaction of metal with solution of salts of other metals

Reactivity series of metals:
- The reactivity of all metals is not the same.
- Oxygen, water, and acids can’t be used to test the reactivity of all metals, because
not all metals react with them. - Displacement reactions (where a metal displaces another metal from its salt
solution) help compare metal reactivity. - Rule:
If metal A displaces metal B from the salt solution of B → metal A is more reactive
than metal B.
From the earlier activity (with iron and copper):
- Iron displaces copper from copper sulphate solution.
- This shows: Iron is more reactive than copper.
Scientists have created the Reactivity Series based on many displacement experiments.
Metals are grouped as:
- Highly reactive metals
- Moderately reactive metals
- Less reactive metals
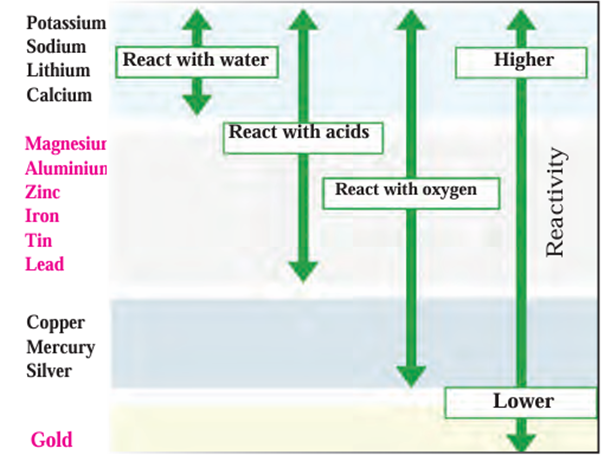
Reactivity series of metals
f. Reaction of metals with non-metals
- Noble gases (like helium, neon, argon) do not take part in chemical reactions
because their outermost electron shells are complete — they are chemically inert. - In metal reactions, metals form cations (positive ions) by losing electrons
(oxidation). - The main driving force behind these reactions is that atoms want to attain the stable
electronic configuration (complete octet) of the nearest noble gas.
o Metals achieve this by losing electrons.
o Nonmetals achieve this by gaining electrons. - Example:
o Sodium (Na, a metal) gives away one electron.
o Chlorine (Cl₂, a nonmetal) takes up one electron.
→ Together, they form the ionic compound NaCl (sodium chloride). - Similarly:
o Magnesium + chlorine → MgCl₂
o Potassium + chlorine → KCl
Chemical properties of non-metals:
- Nonmetals are a collection of elements having less similarity in physical and chemical
properties. - Nonmetals are also called electronegative elements, as they form negatively charged ions by accepting electron.
- Some examples of chemical reactions of nonmetals are as follows.
a. Reaction of nonmetals with oxygen:
- Generally, nonmetals combine with oxygen to form acidic oxides.
- In some cases, neutral oxides are formed.
Examples:
a. Carbon + oxygen (complete combustion):
C + O₂ → CO₂ → acidic oxide (carbon dioxide)
b. Carbon + oxygen (partial combustion):
2C + O₂ → 2CO → neutral oxide (carbon monoxide)
c. Sulfur + oxygen (combustion):
S + O₂ → SO₂ → acidic oxide (sulfur dioxide)
b. Reaction of nonmetals with water:
- Generally, nonmetals do not react with water, except the halogens.
- For example, chlorine on dissolving in water gives the following reaction.
- Cl2 (g) + H2 O(l) → HOCl(aq) + HCl(aq)
c. Reaction of dilute acids with nonmetals:
- Generally, nonmetals do not react with dilute acids, halogens are exception to this.
- For example, chlorine reacts with dilute hydrobromic acid by the following reaction.
- Cl2 (g) + 2HBr (aq) → 2HCl(aq) + Br2 (aq)
d. Reaction of nonmetals with hydrogen:
- Nonmetals react with hydrogen under certain condition (such as proper
temperature, pressure, use of catalyst, etc.) - S + H2 → H2 S
- N2 + 3H2 →2NH3

Ionic compounds:
- The compounds formed from two units, namely cation and anion are called ionic
compounds. - The cation and anion being oppositely charged, there is an electrostatic force of
attraction between them. - The force of attraction between cation and anion is called as the ionic bond.
- The number of cations and anions in a compound and the magnitude of the electric
charge on them is such that the positive and negative charges balance each other. - As a result, an ionic compound is electrically neutral.
- Ionic compounds are crystalline in nature.
- The surfaces of all the particles of a crystalline substance have a definite shape and
are smooth and shiny. - The regular arrangement of ions in the solid ionic compounds is responsible for their
crystalline nature. - The arrangement of ions is different in different ionic compounds, and therefore the
shapes of their crystals are different. - The main factor that determines the general arrangement of ions in a crystal is the
attractive force between oppositely charged ions and the repulsive force between
similarly charged ions. - Because of this the general crystalline structure has negative ions arranged around a
positive ion and positive ions arranged around a negative ion. - Two of the important factors responsible for a certain crystal structure are as
follows.
a. Size of the positively and negatively charged ions.
b. Magnitude of the electrical charge on the ions. - The electrostatic attraction in the neighbouring ions with opposite charges is very
strong. That is why the melting points of ionic compounds are high. - Also, the ionic compounds are hard and brittle.
Properties of ionic compounds:
- The attractive force between the positively and negatively charged ions is strong
Therefore, the ionic compounds exist in solid state and are hard. - The ionic compounds are brittle and can be broken into pieces by applying pressure.
- The intermolecular force of attraction is high in ionic compounds and, large energy is
required to overcome it. Therefore, the melting and boiling points of ionic
compounds are high. - Ionic compounds are water soluble. This is because the water molecules orient in a
particular manner around the ions separated by dissociation process. As a result of
this a new force of attraction is established between the ion and the surrounding
water molecules, replacing the original intermolecular attraction; and aqueous
solutions of ionic compounds are formed. Ionic compounds are however, insoluble in
solvents like kerosene and petrol. This is because unlike water a new attractive force
can not be established in these solvents. - The ionic compounds cannot conduct electricity when in solid state. In this state the
ions cannot leave their places. However, in the fused/molten state they can conduct
electricity, as in this state the ions are mobile. The aqueous solutions of ionic
compounds conduct electricity as they contain the dissociated ions. On passing
current through the solution the ions move to the oppositely charged electrodes. - Due to the electrical conductivity in fused and dissolved state the ionic compound are called electrolytes.
Metallurgy:
The science and technology regarding the extraction of metals from ores and their
purification for the use is called metallurgy.
Occurrence of metals:
- Most metals being reactive do not occur in nature in free state but are found in
combined state as their salts such as oxides, carbonates, sulphides and nitrates. - However, the most unreactive metals that are not affected by air, water and other
natural factors like silver, gold, platinum, generally occur in free state. - The compounds of metals that occur in nature along with the impurities are called
minerals. - The minerals from which the metal can be separated economically are called ores.
- Ores contain many types of impurities such as soil, sand and rocky substances
along with the metal compounds. These impurities are called gangue. - Metals can be extracted from their ores by means of various methods of separation.
- The process of extraction of metal in pure state from the ores is also a part of
metallurgy. - Ores are taken out from the mines and the gangue is usually separated from the ore
at the site itself by various methods. - Then the ores are carried out to the place where metals are produced.
- There metals are extracted in pure form.
- Then metals are further purified by different methods of purification. This entire
process is called metallurgy.
Basic principles of metallurgy:
Pure metal is obtained from the ore by the following stages.
A) Concentration of ores:
- The process of separating gangue from the ores is called concentration of ores.
- In this process the concentration of the compound of the desired metal is increased.
- Various ways are used for this purpose.
- However, exact way to be used depends upon the physical properties of the metal
present in the ores and the gangue. - It also depends upon the reactivity of the metal and the facilities available for the
purification. - Various factors that could be responsible for the environmental pollution are also
considered.
Some general methods for the concentration of ores are as follows.
a. Separation based on gravitation:
The heavy particles of ores can be easily separated from the light particles of gangue by
the gravitational method. The processes to carry out this separation are as follows.
I. Wilfley table method:
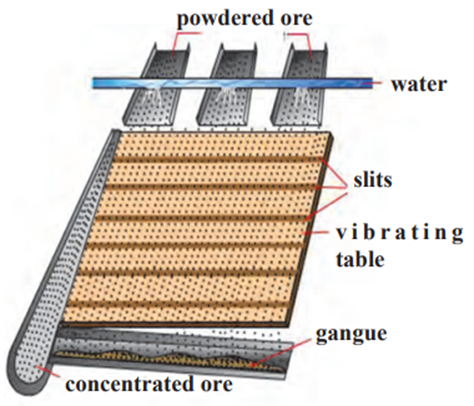
- In this method of separation, the Wilfley table is made by fixing narrow and
thin wooden riffles on inclined surface. - The table is kept vibrating continuously.
- Powdered ore obtained from lumps of the ore using ball mill is poured on the
table and a stream of water is also released from the upper side. - As a result, the lighter gangue particles are carried away along with the
flowing water, while the heavier particles in which proportion of minerals is
more and proportion of gangue is less, are blocked by the wooden riffles and
get collected on the slits between them.
II. Hydraulic separation method:
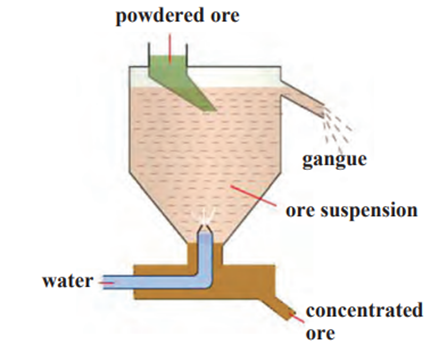
- The hydraulic separation method is based on the working of a mill.
- There is a tapering vessel similar to that used in a grinding mill.
- It opens in a tank-like container that is tapering on the lower side.
- The tank has an outlet for water on the upper side and a water inlet on the lower
side. - Finely ground ore is released in the tank.
- A forceful jet of water is introduced in the tank from the lower side.
- Gangue particles are lighter and therefore they flow out along with the water jet
from the outlet on the upper side of the tank and get collected separately. - At the same time the heavy particles of the ore are collected at the bottom from the
lower side of the tank. - In short, this method is based on the law of gravitation, wherein particles of the
same size are separated by their weight with the help of water.
b. Magnetic separation Method :
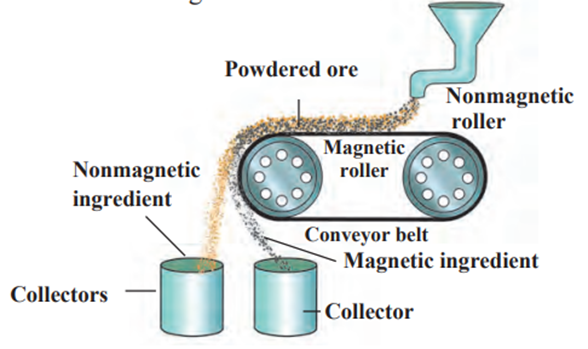
- This method requires an electromagnetic machine.
- The main parts of this machine are two types of iron rollers and the conveyor
belt moving continuously around them. - One of the rollers is nonmagnetic while the other is electromagnetic.
- The conveyor belt moving around the rollers is (nonmagnetic) made up of
leather or brass. - The powdered ore is poured on the conveyor belt near the nonmagnetic
roller. - Two collector vessels are placed below the magnetic roller.
- The particles of the nonmagnetic part in the ore are not attracted towards
the magnetic roller. - Therefore, they are carried further along the belt and fall in the collector
vessel places is away from the magnetic roller. - At the same time the particles of the magnetic ingredients of the ore stick to the magnetic roller and therefore fall in the collector vessel near the magnetic roller.
- In this way the magnetic and nonmagnetic ingredients in the ore can be
separated depending on their magnetic nature. - For example, cassiterite is a tin ore.
- It contains mainly the nonmagnetic ingredient stannic oxide (SnO2 ) and the
magnetic ingredient ferrous tungstate (FeWO4 ). - These are separated by the electromagnetic method.
c. Froth floatation method:
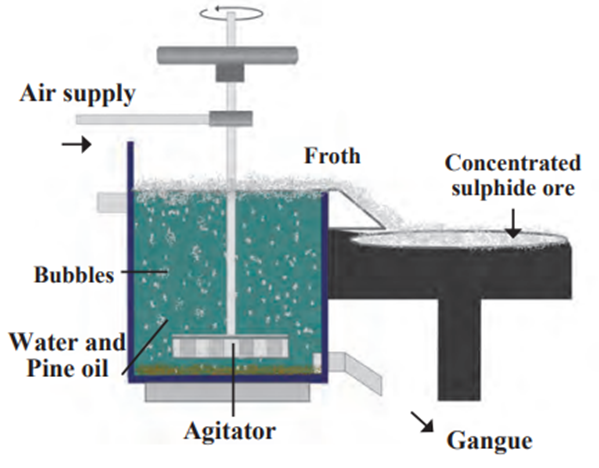
- The froth floatation method is based on the two opposite properties, hydrophilic and
hydrophobic, of the particles. - Here the particles of the metal sulphides, due to their hydrophobic property, get
wetted mainly with oil, while due to the hydrophilic property the gangue particles
get wetted with water. - By using these properties certain ores are concentrated by froth floatation method.
- In this method the finely ground ore is put into a big tank containing ample amount
of water. - Certain vegetable oil such as pine oil, eucalyptus oil is added in the water for the
formation of froth. - Pressurised air is blown through the water.
- There is an agitator rotating around its axis in the centre of the floatation tank.
- The agitator is used as per the requirement.
- Bubbles are formed due to the blown air.
- Due to agitation a foam is formed from oil, water and air bubbles together, due to the agitating.
- This foam rises to the surface of water and floats. That is why this method is called
froth floatation process. - Particles of certain sulphide ore float with the foam on water as they preferentially
get wetted by the oil. For example, this method is used for the concentration of zinc
blend (ZnS) and copper pyrite (CuFeS2)
d. Leaching:
- The first step in the extraction of the metals aluminium, gold and silver from their
ores is the method of leaching. - In this method the ore is soaked in a certain solution for a long time.
- The ore dissolves in that solution due to a specific chemical reaction.
- The gangue, however, does not react and therefore does not dissolve in that
solution. - So it can be separated.
- For example, concentration of bauxite, the aluminium ore, is done by leaching
method. - Here bauxite is soaked in aqueous NaOH or aqueous Na2CO3which dissolves the
main ingredient alumina in it.
B) Extraction of metals:
a) Extraction of reactive metals:
- The metals at the top of the reactivity series are highly reactive.
- Their reactivity decreases down the series. For example, potassium, sodium,
aluminium are reactive metals. - Reactive metals have large capacity to form cations by losing the electrons in their
outermost shell. For example, reactive metals react vigorously with dilute acids to
give hydrogen gas. - Highly reactive metals burn by reacting with oxygen from air at room temperature.
- Their extraction has to be done by electrolytic reduction. For example, the metals
sodium, calcium and magnesium are obtained by electrolysis of their molten chloride
salts. - In this process metal is deposited on the cathode while chlorine gas is liberated at
the anode. - The electrode reactions during the electrolysis of molten sodium chloride to get
metallic sodium are as shown below.
Cathode Reaction:
Na⁺ + e⁻ → Na
Anode reaction:
2Cl⁻ → Cl₂ + 2e⁻

Extraction of Aluminium:
- Aluminium Symbol : Al
- Colour : Silver white
- Atomic number : 13
- Electronic configuration: 2, 8, 3
- Valency : 3
- Aluminium being reactive metal does not occur in nature in free state.
- Aluminium is the third highly abundant element in the earth crust after oxygen and
silicon. - Aluminium is extracted from its ore bauxite (Al2O3.nH2O). Bauxite contains 30% to
70% of (Al2O3 and remaining part is gangue. - It is made up of sand, silica, iron oxide etc.
- There are two steps in the extraction of aluminium.
A) Concentration of bauxite ore:
- Bauxite is the main ore of aluminium.
- Silica (SiO2), ferric oxide (Fe2O3) and titanium oxide (TiO2 ) are the impurities
present in bauxite. - Separation of these impurities is done by leaching process using either Bayer’s
method or Hall’s method. - In both these methods finally the concentrated alumina is obtained by calcination.
- In the Bayer’s process the ore is first ground in a ball mill.
- Then it is leached by heating with concentrated solution of caustic soda (NaOH) at
140 to 150℃ under high pressure for 2 to 8 hours in a digester. - Aluminium oxide being amphoteric in nature, it reacts with the aqueous solution of
sodium hydroxide to form water soluble sodium aluminate. This means that bauxite
is leached by sodium hydroxide solution.
Al2O3.2H2O(s)+2NaOH(aq)→2NaAlO2(aq)+3H2O(l) - The iron oxide in the gangue does not dissolve in aqueous sodium hydroxide.
- It is separated by filtration.
- However, silica in the gangue dissolves in aqueous sodium hydroxide to form water
soluble sodium silicate. - Aqueous sodium aluminate is diluted by putting in water and is cooled to 50℃.
- This results in precipitation of aluminium hydroxide.

- In the Hall’s process the ore is powdered and then leached by heating with aqueous
sodium carbonate in the digester to form water soluble sodium aluminate. - Then the insoluble impurities are filtered out.
- The filtrate is warmed and neutralised by passing carbon dioxide gas through it.
- This results in the precipitation of aluminium hydroxide.
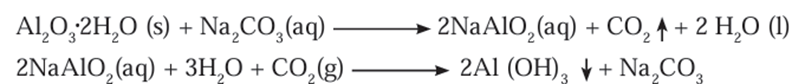
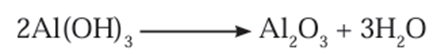
- The precipitate of Al(OH)3 obtained in both, Bayer’s and Hall’s processes is filtered,
washed, dried and then calcined by heating at 1000℃ to obtain alumina.
B) Electrolytic reduction of alumina:
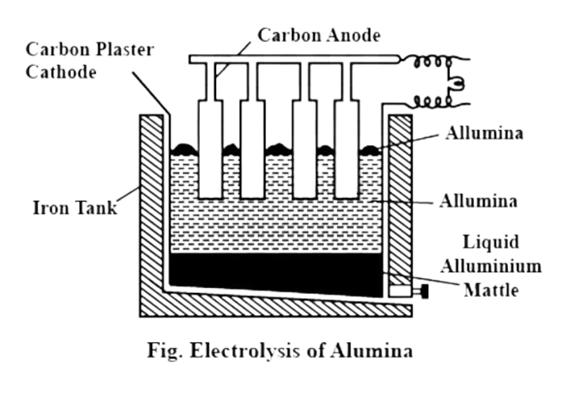
a) 1. In this method electrolysis of molten mixture of alumina (melting point > 2000
0C) is done in a steel tank.
- The tank has a graphite lining on the inner side.
- This lining does the work of a cathode.
- A set of graphite rods dipped in the molten electrolyte works as anode.
Cryolite (Na3AlF6) and fluorspar (CaF2) are added in the mixture to lower its
melting point up to 1000℃.
b) 1. Aluminium is deposited on the cathode on passing electric current.
- Molten aluminium being heavier than the electrolyte, is collected at the
bottom of the tank. - It is taken out from there from time to time, Oxygen gas is liberated at the
anode. - The liberated oxygen reacts with the anodes to form carbon dioxide gas.
- The anodes have to be changed from time to time as they get oxidised during
the electrolysis of alumina.
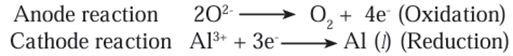
Extraction of moderately reactive metals:
- The metals in the middle of the reactivity series such as iron, zinc, lead, copper are
moderately reactive. - Usually they occur in the form of their sulphide salts or carbonate.
- It is easier to obtain metals from their oxides rather than sulphides or carbonates.
- Therefore, the sulphide ores are strongly heated in air to transform them into
oxides. This process is called roasting. - Carbonate ores are strongly heated in a limited supply of air to transform them
into oxides. This process is called calcination. - The following reactions occur during roasting and calcination of zinc ore.
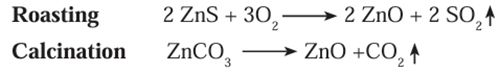
- The zinc oxide so obtained is reduced to zinc by using suitable reductant such as
carbon.

- Apart from carbon, reactive metals such as sodium, calcium, aluminium are also used
as reducing agent for the reduction of metal oxide to obtain the metal. - This is because these metals displace a moderately reactive metal from its
compound. - For example, when manganese dioxide is ignited with aluminium powder the
following reaction takes place.
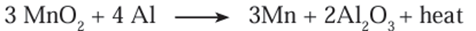
- The heat evolved in the above reaction is so large that the metal is formed in the
molten state. - Another similar example is the thermit reaction. Here, iron oxide reacts with
aluminium to form iron and aluminium oxide.
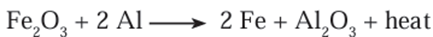

Extraction of less reactive metals:
- The metals at the bottom of the reactivity series of metals are less reactive.
- That is why they are found in free state in nature.
- For example gold, silver, platinum.
- The reserves of copper in free state are very few.
- Presently copper is found mainly in the form of Cu2S.
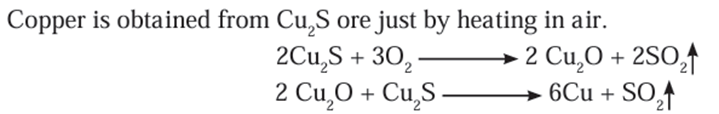
Refining of metals:
- Metals obtained by the various reduction processes described above are not very
pure. - They contain impurities.
- The impurities need to be separated to obtain pure metal.
- Electrolysis method is used to obtain pure metals from impure metals.
Corrosion of metals:
Corrosion is a major problem that causes damage to metals over time when exposed to air
and moisture. It can lead to big financial losses, especially due to rusting of iron.
Rusting of Iron:
o Iron reacts with moist air.
o A reddish-brown substance is formed on its surface.
o This substance is rust, chemically known as Fe₂O₃·H₂O (hydrated iron(III)
oxide).
- Copper (Patination):
o Carbon dioxide in moist air reacts with the surface of copper.
o A greenish layer of copper carbonate (CuCO₃) forms.
o This layer causes the copper to lose its shine.
o This process is called patination. - Silver:
o Silver reacts with hydrogen sulphide (H₂S) in air.
o A blackish layer of silver sulphide (Ag₂S) forms.
o This makes silver items like ornaments and idols look tarnished. - Aluminium:
o Aluminium reacts with oxygen in air.
o A thin layer of aluminium oxide (Al₂O₃) forms.
o This layer protects the aluminium from further corrosion.
Prevention of corrosion:
- General Idea:
o Corrosion (especially rusting of iron) can be reduced by blocking the metal’s
contact with moisture and air. - Method 1: Coating the Metal Surface
o A layer of some protective substance is fixed on the metal.
o This stops air and moisture from reaching the metal and prevents reactions. - Method 2: Applying Paint, Oil, Grease, or Varnish
o Iron articles are often protected by coating them with paint, oil, grease, or
varnish.
o This method is temporary.
o If the layer is scratched, air and moisture reach the metal, and rusting starts
under the paint.
Why do new iron sheets appear shiny?
New iron sheets are coated with a protective layer (usually zinc) to prevent
corrosion and give them a shiny appearance.
Using Non-Corrodible Metals for Protection:
1. A layer of non-corrodible metal (like zinc, tin, or chromium) can be applied to a
corrodible metal (like iron).
2. This is a more effective and long-lasting method.
3. Examples include:
o Galvanization – coating iron with zinc.
o Tinning – coating with tin.
o Electroplating – applying a thin layer of another metal using electricity.
Galvanizing:
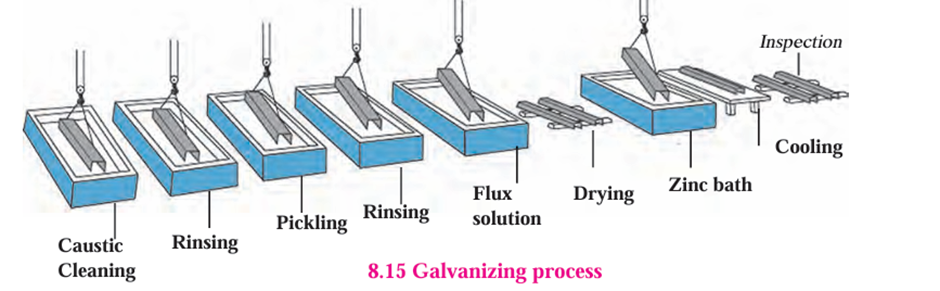
- In this method a thin layer of zinc is applied to prevent corrosion of iron or steel.
- For example, shining iron nails, pins, etc. In this method corrosion of zinc occurs first
because zinc is more electropositive than iron. - After a few rainy seasons the zinc layer goes away and the inner iron gets exposed.
- Then iron starts rusting.
Tinning:
- In this method a layer of molten tin is deposited on metals. We call this as ‘kalhaee’.
- A greenish layer forms on the surface of a copper or brass vessel.
- This greenish layer is poisonous.
- If buttermilk or curry is placed in such a vessel it gets spoiled.
- Tinning is done to prevent all such damages.
Anodization:
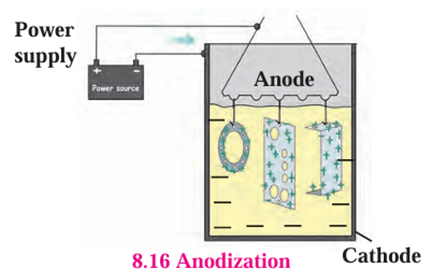
- In this method metals like copper, aluminium are coated with a thin and strong layer
of their oxides by means of electrolysis. - For this the copper or aluminium article is used as anode.
- As this oxide layer is strong and uniform all over the surface, it is useful for
prevention of the corrosion of the metal. For example, when aluminium is anodised,
the thin layer of aluminium oxide is formed. - It obstructs the contact of the aluminium with oxygen and water.
- This prevents further oxidation.
- This protection can be further increased by making the oxide layer thicker during the
anodization.
Electroplating:
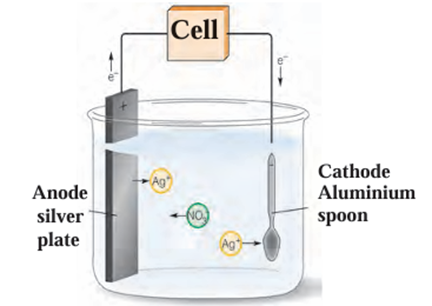
- In this method a less reactive metal is coated on a more reactive metal by
electrolysis. - Silver plated spoons, gold plated ornaments are the examples of electroplating.
Alloying:
- Majority of the metallic substances used presently are in the form of alloys.
- The main intention behind this is to decrease the intensity of corrosion of metals.
- The homogenous mixture formed by mixing a metal with other metals or nonmetals
in certain proportion is called an alloy. For example, bronze is an alloy formed from
90% copper and 10 % tin. - Bronze statues do not get affected by sun and rain.
- Stainless steel does not get stains with air or water and also does not rust.
- It is an alloy made from 74% iron, 18% chromium and 8% carbon.
- In recent times various types of alloys are used for minting coins.

Solutions for the exercises, unsolved numerical problems, and solved examples will soon be available for download in convenient PDF format at very affordable prices. Our notes are carefully crafted, accurate, and aligned with board requirements to help you succeed.
