Std 10 Science Part 1 Chapter 2 Periodic Classification of Elements Notes Maharashtra Board
Class 10 Science Part 1 Chapter 2 Periodic Classification of Elements Notes Maharashtra Board

Classification of elements:
- All the atoms of an element are of only one type.
- Today 118 elements are known to the scientific world.
- In the initial classification elements were classified into the groups of metals and
nonmetals. - Later on another class of elements called metalloids was noticed.
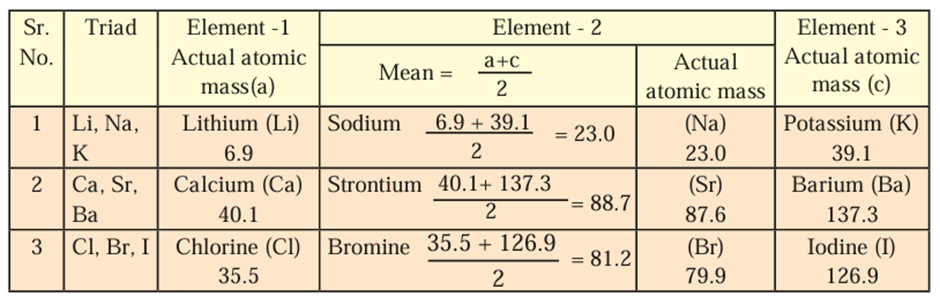
Dobereiner’s Triads:
- Dobereiner suggested that properties of elements are related to their atomic
masses. - He made groups of three elements each, having similar chemical properties and
called them triads. - He arranged the three elements in a triad in an increasing order of atomic mass and
showed that the atomic mass of the middle element was approximately equal to the
mean of the atomic masses of the other two elements. - However, all the known elements could not be classified into the Dobereiner’s triads.
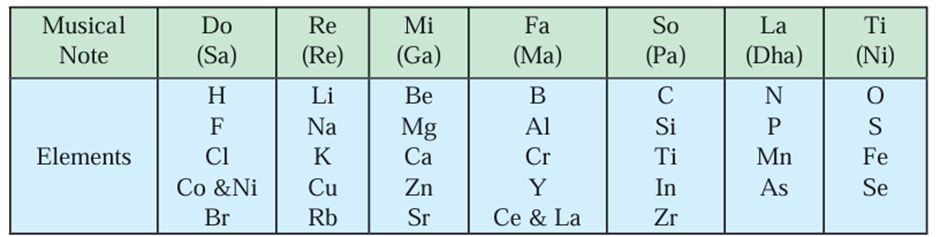
Newlands’ Law of Octaves:
- John Newlands correlated the atomic masses of elements to their properties in a
different way. - In the year 1866 Newlands arranged the elements known at that time in an
increasing order of their atomic masses. - It started with the lightest element hydrogen and ended up with thorium.
- He found that every eighth element had properties similar to those of the first.
- For example, sodium is the eighth element from lithium and both have similar
properties. - Also, magnesium shows similarity to beryllium and chlorine shows similarity with
fluorine. - Newlands compared this similarity with the octaves in music. He called the
similarity observed in the eighth and the first element as the Law of octaves. - Many limitation were found in Newlands’ octaves. This law was found to be applicable only up to calcium.
- Newlands fitted all the known elements in a table of 7 X 8 that is 56 boxes.
- Newlands placed two elements each in some boxes to accommodate all the known elements in the table.
- For example, Co and Ni, Ce and La. Moreover, he placed some elements with different properties under the same note in the octave.
- For example, Newlands placed the metals Co and Ni under the note ‘Do’ along with halogens, while Fe, having similarity with Co and Ni, away from them along with the nonmetals O and S under the note ‘Ti’.
- Also, Newlands’ octaves did not have provision to accommodate the newly discovered elements.
- The properties of the new elements discovered later on did not fit in the Newlands’ law of octaves.

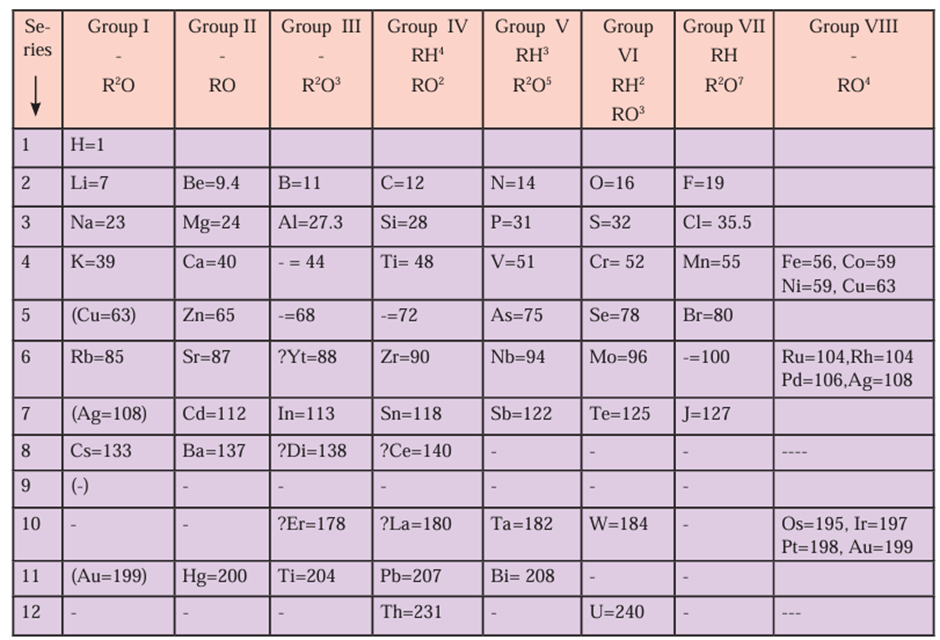
Mendeleev’s Periodic table:
- Mendeleev’s periodic table is the most important step in the classification of
elements. - Mendeleev considered the fundamental property of elements, namely, the atomic
mass, as standard and arranged 63 elements known at that time in an increasing
order of their atomic masses. - Then he transformed this into the periodic table of elements in accordance with
the physical and chemical properties of these elements. - Mendeleev organized the periodic table on the basis of the chemical and physical
properties of the elements. - These were the molecular formulae of hydrides and oxides of the elements, melting points, boiling points and densities of the elements and their hydrides and oxides.
- Mendeleev found that the elements with similar physical and chemical properties repeat after a definite interval.
- On the basis of this finding Mendeleev stated the following periodic law.
- Properties of elements are periodic function of their atomic masses.
- The vertical columns in the Mendeleev’s periodic table are called groups while the horizontal rows are called periods.
Merits of Mendeleev’s periodic table:
Mendeleev’s periodic table demonstrates the following merits.
- Atomic masses of some elements were revised so as to give them proper place in
the periodic table in accordance with their properties. For example, the previously
determined atomic mass of beryllium, 14.09, was changed to the correct value 9.4,
and beryllium was placed before boron.
- Mendeleev kept vacant places in the periodic table for elements not discovered till
then. Three of these unknown elements were given the names eka-boron, eka
aluminum and eka-silicon from the known neighbours and their atomic masses were indicated as 44, 68 and 72, respectively. Not only this but their properties were also predicted. Later on these elements were discovered and named as scandium (Sc), gallium (Ga) and germanium (Ge) respectively. The properties of these elements matched well with those predicted by Mendeleev. - There was no place reserved for noble gases in Mendeleev’s original periodic table.
However, when noble gases such as helium, neon and argon were discovered
towards the end of nineteenth century, Mendeleev created the ‘ zero’ group without
disturbing the original periodic table in which the noble gases were fitted very well.
Demerits of Mendeleev’s periodic table:
- The whole number atomic mass of the elements cobalt (Co) and nickel (Ni) is the
same. Therefore there was an ambiguity regarding their sequence in Mendeleev’s
periodic table. - Isotopes were discovered long time after Mendeleev put forth the periodic table. As
isotopes have the same chemical properties but different atomic masses, a challenge
was posed in placing them in Mendeleev’s periodic table. - When elements are arranged in an increasing order of atomic masses, the rise in
atomic mass does not appear to be uniform. It was not possible, therefore, to predict
how many elements could be discovered between two heavy elements. - Position of hydrogen : Hydrogen shows similarity with halogens (group VII). For
example, the molecular formula of hydrogen is H2 while the molecular formulae of
fluorine and chlorine are F2 and Cl2 , respectively. In the same way, there is a
similarity in the chemical properties of hydrogen and alkali metals (group I). There is
a similarity in the molecular formulae of the compounds of hydrogen alkali metals
(Na, K, etc.) formed with chlorine and oxygen. On considering the above properties it
can not be decided whether the correct position of hydrogen is in the group of alkali
metals (group I) or in the group of halogens (group VII).
Modern Periodic Law:
- Henry Moseley demonstrated, with the help of the experiments done using X-ray
tube, that the atomic number (Z) of an element corresponds to the positive charge
on the nucleus or the number of the protons in the nucleus of the atom of that
element. - This revealed that ‘atomic number’ is a more fundamental property of an element
than its atomic mass. - Accordingly the statement of the modern periodic law was stated as follows:
Properties of elements are a periodic function of their atomic numbers.

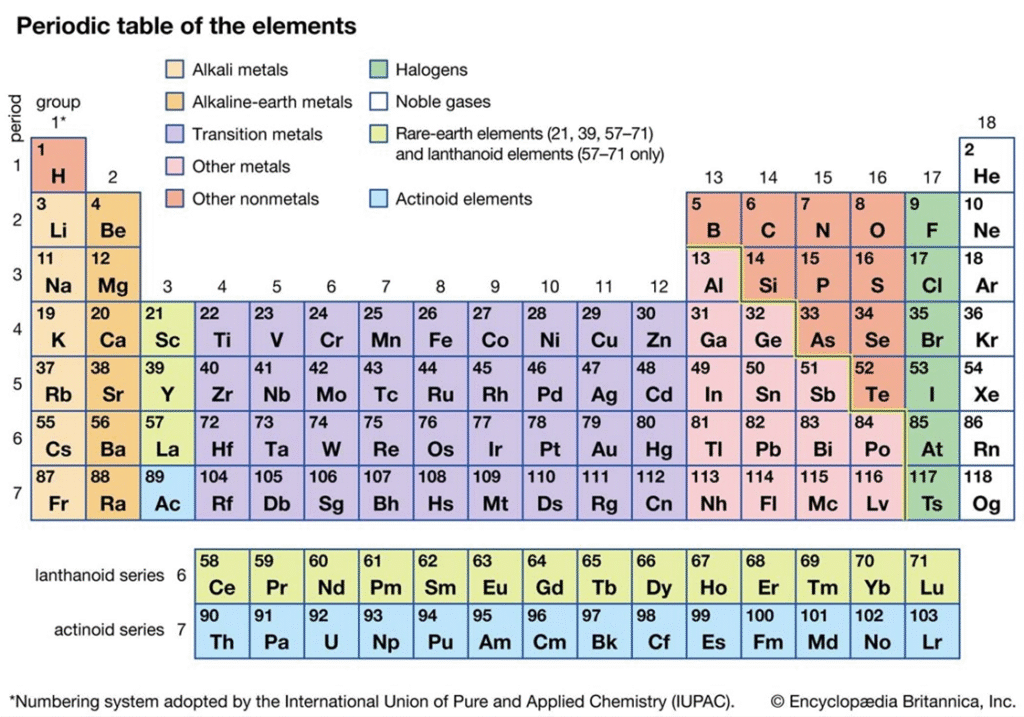
Modern periodic table : long form of the periodic table:
Definition:
The modern periodic table is an arrangement of elements in increasing order
of their atomic numbers.
Accurate Prediction of Properties:
Element properties can be predicted more accurately based on their position
in the modern periodic table.
Also Known As:
It is also called the long form of the periodic table.
Basis of Arrangement:
Elements are arranged according to their atomic numbers, not atomic
masses.
Correction of Drawbacks:
This arrangement removes most of the drawbacks found in Mendeleev’s
periodic table.
Exception – Hydrogen:
The position of hydrogen is still ambiguous in the modern periodic table.
Relation to Electronic Configuration:
The electronic configuration of an atom is related to its atomic number (i.e.,
number of electrons = atomic number).
Shell Distribution:
Electrons are distributed in shells around the nucleus according to the
atomic number.
Pattern in Periodicity:
The periodicity (repeating patterns in properties) is clearly understood
through electronic configurations and their relation to atomic number.
Structure of the Modern Periodic Table:
Periods (Horizontal Rows):
The periodic table has 7 horizontal rows called periods, numbered 1 to 7.
Groups (Vertical Columns):
There are 18 vertical columns called groups, numbered 1 to 18.
Element Boxes:
The arrangement of periods and groups forms 118 boxes, each representing
an element with its atomic number indicated.
Separate Series:
Two rows at the bottom are shown separately:
1. Lanthanide series
2. Actinide series
3. These belong to the f-block.
Total Elements:
The periodic table now includes all 118 known elements, and it is completely
filled with no vacant positions.
Block Division:
The periodic table is divided into four blocks based on electron configuration:
- s-block: Groups 1 and 2
- p-block: Groups 13 to 18
- d-block: Groups 3 to 12 (Transition elements)
- f-block: Lanthanides and actinides
Transition Elements:
d-block elements are referred to as transition elements.
Zig-Zag Line:
A zig-zag line is drawn in the p-block, dividing:
1. Metals (left side)
2. Nonmetals (right side)
3. Metalloids (along the border of the line)
Element Placement:
Each box in the periodic table represents a single element and shows its
atomic number.

Modern periodic Table and electronic Configuration of Elements:
- Neighbouring elements in a period have similar but slightly different properties.
- Elements far apart in a period show big differences in their properties.
- Elements in the same group have similar properties and show a gradual change
(gradation) from top to bottom. - These patterns in groups and periods happen because of the electronic
configuration of the elements. - The electronic configuration of an element decides its position in the periodic table:
a) Which group it belongs to.
b) Which period it belongs to.
Groups and electronic configuration:
- All elements in the same group have the same number of valence electrons
(electrons in the outermost shell). - Group 1 (Alkali metals) – All elements have 1 valence electron.
- Group 2 (Alkaline earth metals) – Elements like Be, Mg, Ca have 2 valence
electrons. - Group 17 (Halogens) – Elements like F and Cl have 7 valence electrons.
- As you go down a group, one more electron shell is added each time.
- The outermost electronic configuration stays similar in a group, but the number of
shells increases.
Periods and electronic configuration:
- In a period, the number of valence electrons increases by one as you go left to
right. - All elements in the same period have the same number of electron shells.
- Example:
a) 2nd Period (Li to Ne): All elements have 2 shells (K and L).
b) 3rd Period (Na to Ar): All elements have 3 shells (K, L, and M). - From left to right in a period:
a) Atomic number increases by 1.
b) Valence electrons increase by 1.
c) Electrons are added to the same shell. - At the start of a new period, a new shell begins to fill with electrons.
- The number of elements in a period is based on:
a) Electron capacity of shells
b) Octet rule (maximum 8 electrons in the outer shell for stability) - Example of number of elements:
a) 1st Period: 2 elements b) 2nd Period: 8 elements c) 3rd Period: 8 elements - Chemical reactivity of an element depends on:
a) Number of valence electrons
b) Which shell these valence electrons are in - The position of an element in the modern periodic table helps us understand:
a) Its electronic configuration
b) Its reactivity
c) Its properties - The modern periodic table is very useful for studying elements and predicting their
behaviour.
Periodic trends in the modern periodic table:
- Periodic Trends:
a) When comparing elements in a period (row) or a group (column), certain
patterns are seen in their properties. These are called periodic trends. - In this standard, we study trends in 3 properties:
a) Valency
b) Atomic size
c) Metallic and non-metallic character
Valency:
Valency is the combining capacity of an element.
a) It is decided by the number of valence electrons (electrons in the outermost
shell).
Trend Across a Period (Left to Right):
- As you go from left to right in a period, the valency first increases and then
decreases.
Example:
a) Period 2:
Li (1), Be (2), B (3), C (4), N (3), O (2), F (1), Ne (0)
b) Valency increases from 1 to 4, then decreases back to 0.
c) Same pattern is seen in Period 3:
Na (1), Mg (2), Al (3), Si (4), P (3), S (2), Cl (1), Ar (0)
Trend Down a Group (Top to Bottom):
As you go down a group, the valency stays the same.
Example:
a) Group 1: Li, Na, K → All have valency 1
b) Group 2: Be, Mg, Ca → All have valency 2
c) Group 18: He, Ne, Ar → All have valency 0 (inert gases)

Atomic size:
Atomic Radius:
a) It is the distance between the nucleus and the outermost shell of an atom.
b) It tells us the size of an atom.
c) It is measured in picometers (pm)
(1 pm = 10⁻¹² meters)
Trend Across a Period (Left to Right):
As you move left to right in a period, atomic radius decreases.
a) Atomic number increases (more protons in the nucleus).
b) Electrons are added to the same shell.
c) The increased nuclear charge pulls electrons closer to the nucleus.
d) So, atom becomes smaller.
Trend Down a Group (Top to Bottom):
As you move down a group, atomic radius increases.
a) A new electron shell is added at each step down.
b) This increases the distance between nucleus and outermost electron.
c) So, atoms become larger, even though nuclear charge also increases.
Metallic- Nonmetallic Character:
- Metals (like sodium, magnesium) are on the left side of the periodic table.
- Nonmetals (like sulfur, chlorine) are on the right side.
- Metalloids (like silicon) are found along the zig-zag line between metals and
nonmetals.
What is Metallic Character?
Metallic character is the tendency of an atom to lose electrons and form positive
ions (cations).
a) This is also called electropositivity.
b) Metals have 1 to 3 valence electrons.
c) They lose these electrons easily because:
d) Valence electrons are far from the nucleus, especially in bigger
atoms.
e) Attraction from the nucleus is weak due to inner electron shielding.
Trend of Metallic Character:

Why metallic character increases down a group?
a) New shells are added, so outer electrons are farther from the nucleus.
b) Less nuclear pull, so it’s easier to lose electrons.
Why metallic character decreases across a period?
a) Nuclear charge increases, pulling electrons closer.
b) Harder to lose electrons.
What is Nonmetallic Character?
Nonmetallic character is the tendency to gain electrons and form negative ions
(anions).
a) This is also called electronegativity.
b) Nonmetals have more valence electrons (5 to 7).
c) They can easily complete their octet by gaining electrons.
Trend of Nonmetallic Character:

Why nonmetallic character increases across a period?
a) Atomic size decreases, and nuclear charge increases.
b) Atoms pull electrons more strongly.
Why nonmetallic character decreases down a group?
a) Atoms become larger with more shells.
b) Weaker pull on incoming electrons.
Gradation in Halogen Family:
1. The group 17 contains the members of the halogen family.
2. All of them have the general formula X2 .
3. A gradation is observed in their physical state down the group.
4. Thus, fluorine (F2 ) and chlorine (Cl2 ) are gases, bromine (Br2 ) is a liquid while
iodine (I2 ) is a solid

Solutions for the exercises, unsolved numerical problems, and solved examples will soon be available for download in convenient PDF format at very affordable prices. Our notes are carefully crafted, accurate, and aligned with board requirements to help you succeed.
